“Killer T cell attacking cancer” posted by Cambridge University, Feb 3, 2012. T cell shown in green, cancer cell shown in blue.
The immune system has a very difficult job. It has to find the metaphorical needle in the haystack and distinguish the very few infected and cancerous cells from the billions of normal cells throughout the body. To achieve this very difficult task, T cells, the workhorse cells for surveillance, have to physically crawl over and interact with target cells which in turn display snippets of their proteins on their plasma membrane. The recognition process is mediated by T cell receptors (TCRs) that signal once they bind antigens on target cells. TCR-rich microvilli push and pull on the apposing cell membrane as the T cell scans for antigens. This is clear from the video shown to the right. Videos like this prompted us to ask the following questions: Is the TCR-antigen complex experiencing pushing and pulling forces? What is the magnitude and duration of the force? How does force influence immune response? Many others in the field had postulated and demonstrated that the TCR-antigen bond is sensitive to force, but for us we were interested in measuring the intrinsic forces generated by the T cell and experienced by the antigen. We also wanted to understand the process of mechanosensing and mechanotransduction. Our approach has focused on developing tools to measure and in some cases control forces and then to record signaling outcomes. For reviews on this topic please see the following papers: DNA Nanotechnology for Investigating Mechanical Signaling in the Immune System (link), DNA Nanotechnology as an Emerging Tool to Study Mechanotransduction in Living Systems (link), and Mechanical Control of Antigen Detection and Discrimination by T and B Cell Receptors (link).
DNA-based MTFM to visualize TCR forces
Our work in this area started when we used molecular tension probes (see previous section on mechanobiology probes) to investigate TCR forces (PNAS 2016). We discovered that TCRs transmit piconewton-scale forces to their antigens (for CD8+ OT-1 cells 12pN>F>19pN). The force probe was comprised a force-responsive DNA hairpin structure flanked by a FRET pair which generates a fluorescent “on” signal upon force exertion. Our foray in this field was very exciting as we were able to demonstrate that the magnitude of these forces was highly dependent on kinase signaling and co-receptor engagement with the number of mechanical events detected increasing with antigen potency. Additionally, we found that these forces aid T cells in recognizing and initiating TCR stimulation upon engagement with their cognate antigens (PNAS 2016).
DNA tension probes for mechanical information storage
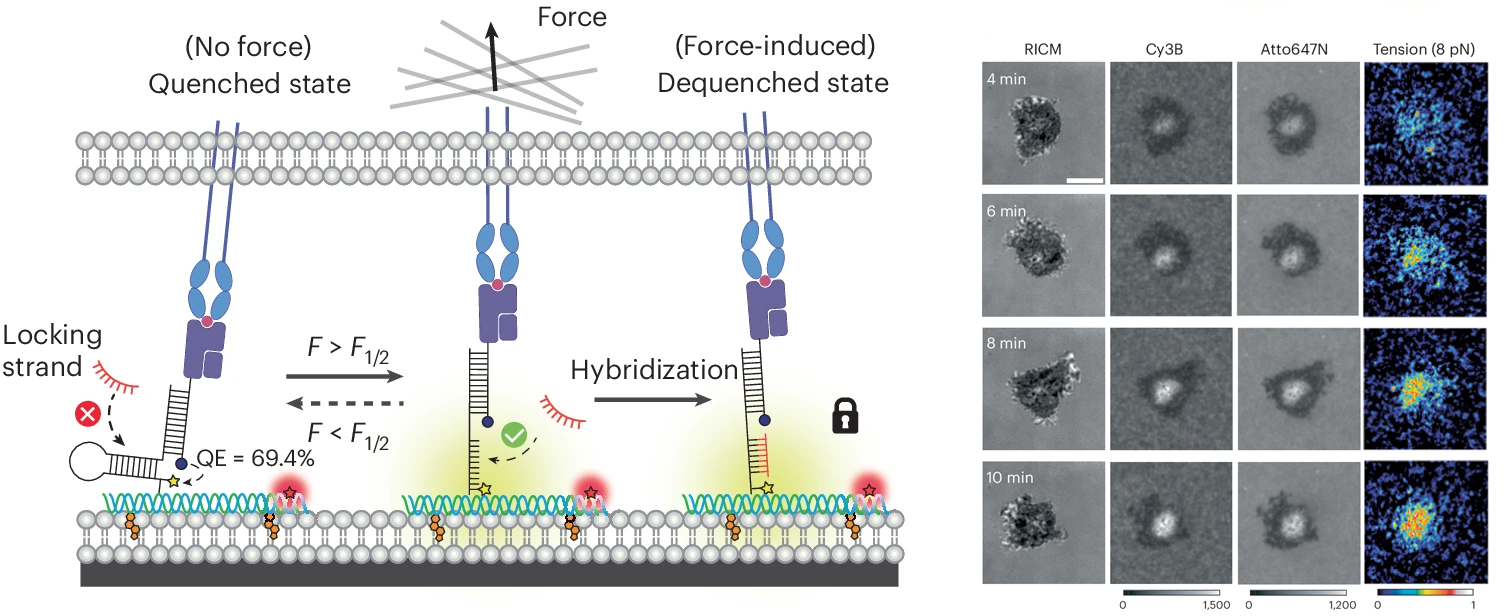
While we were able to visualize TCR-mediated forces with our initial DNA-based tension probe, often times the observed signal was relatively weak as we studied the interaction between TCRs and pMHCs, the natural ligand for the TCR. This is due to the transiency of this interaction; previous work has shown that most of these interactions have a bond liftetimes in the millisecond range. To capture these highly transient interactions, we developed a technique that allows for mechanically unfolded tension probes to be locked in their open state. This allows tension signal to accumulate over time and enables visualization of mechanical events that are not visible by observing a real time snapshot of a small population of unfolded probes (PNAS 2019). An important outcome of this study is the development of a new class of probes that trigger a chemical response (DNA binding) in response to a precise pN mechanical event. This is the basis for many subsequent technologies described below.
T cell co-receptor forces enhance T cell activation
In addition to the TCR-pMHC interaction, T cells engage a number of ligands on antigen presenting cells that promote either T cell activation or immune suppression. A key co-stimulatory interaction that promotes T cell activation by promoting cellular adhesion is the engagement of ICAM-1 by LFA-1 on the T cell surface. Using spectrally encoded DNA tension sensors, we visualized TCR and LFA-1 forces simultaneously as a T cell interacts with a substrate decorated with cognate pMHC and ICAM-1. Additionally, we discovered that tension between LFA-1 and ICAM-1 must be sustained to fully potentiate a T cell response. This was the first direct observation of LFA-1 exerting force onto ICAM-1 and analysis of the role that these forces play in T cell activation (Science Advances 2022).
TCR-pMHC serial mechanical engagement enhance T cell activation
We next became curious as to whether a single mechanical event is sufficient to trigger the TCR and mediate its response or whether the TCR needs repeated mechanical sampling of its antigen. In other words, does a single “handshake” provide the needed information to mount an immune response or would the TCR benefit from having multiple handshakes? This idea ties to the serial engagement model that proposes that a single antigen is sampled repeatedly by different TCRs to mediate an ultrasensitive response. So we were hoping to reveal the interplay between TCR mechanosensing and serial engagement. We designed a probe that “self-destructs” after a defined time following mechanical triggering. The FUSE probe recruits a nuclease enzyme only if mechanically triggered and the timing of the self-destruction is set by the enzyme concentration. This allowed us to study the relationship between TCR-antigen force duration and T cell stimulation and the results demonstrated that serial mechanical engagement enhances T cell activation. Therefore, it meant that the TCR takes advantage of repeated pN mechanical sampling to boost the levels of T cell stimulation. The work was the first demonstration of the potential cooperation between the mechanosensor and serial engagement models for T cell activation (JACS 2024).
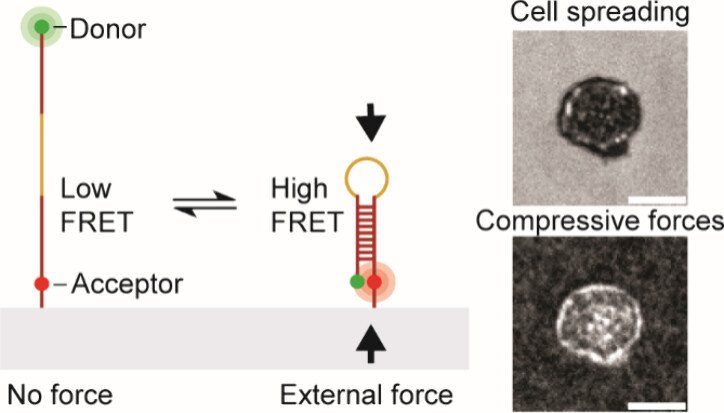
T cells exert compressive forces upon antigen engagement
While previous work robustly characterized tensile “pulling” forces between various T cell receptors and their ligands, we became curious as to whether the T cell “pushes” on the target cell. To address this question, we developed a molecular compression probe that allows compressive forces applied by T cells (and other cell types) to be visualized using FRET-based fluorescence microscopy. The key innovation here was to screen for DNA hairpins sequences that are pseudostable – at the verge of folding – but not fully folded at equilibrium. However, when an external compressive force is applied to the probe, then this encourages the hairpin to fold, and shifts the equilibrium toward the folded state. Effectively, this is akin to a crowding sensor. The probe generates high FRET signal only upon folding and the image to the right shows a map of T cell pushing forces applied to the substrate. This work demonstrates the first probe that maps molecular compressive forces and has been used to study molecular compressive forces exerted by T cells and platelets (JACS 2024).
DNA origami tension sensors to quantify forces at cell-cell interface
We next worked on finding methods of anchoring the DNA probes onto phospholipid membranes – which better mimic the physiological environment of TCR-antigen recognition. We particularly needed to keep probes at set distances (10-20 nms) away to avoid energy transfer processes probe-to-probe. We achieved this by creating DNA origami tension sensors (DOTS) that measure TCR-mediated forces in lipid membranes. DOTS can be tethered to virtually any lipid membrane, thus allowing one to study mechanotransduction at cell-cell junctions. DOTS are comprised of a rectangular nanosheet that presents a single force sensor on one face and cholesterol moieties for anchoring to membranes on the other face. We anchor DOTS to supported lipid membranes (SLBs) as well as live cell plasma membranes to present specific ligand and record the forces experienced by these ligands. The DOTS platform overcomes a long-standing limitation in this field, which is that lateral clustering of tension sensors leads to intermolecular FRET that creates artifacts and hinders quantitation of forces at fluid interfaces. We show unambiguously that T cells transmit >5 pN forces to their cognate antigens within live cell-cell junctions. We identify the source of TCR forces during initial antigen engagement and confirm that force is dependent on the nanometer height of the antigen and also on actin polymerization. We also demonstrate the potential for DOTS to be used in antigen screening by using spherical microparticles amenable to flow cytometry analysis of TCR-pMHC tension. This allows one to potentially quantify of hundreds of thousands T-cell mechanical properties within minutes and can be leveraged as a tool to screen antigen based on its potency to trigger biomechanical signaling (Nature Nanotech. 2024).
Future work
We are just starting to understand the role of molecular force in immune response and there remains a wide array of remaining questions. How does the cytoskeleton transmit force to the antigen? What is the precise relationship between force and bond lifetime? How are force magnitudes tuned? How does the structure of the TCR-antigen complex change with the application of pN forces? Does the co-receptor also experience forces? We recently showed that PD-1 and other co-receptors also transmit force. What are the role of these forces in co-receptors? And finally, as we “peel away the layers of the onion” and understand the molecular mechanisms of mechanotransduction in immune responses, we continually seeking to identify new diagnostics and therapeutics that can be realized by taking advantage of this new understanding.