RNA degraders pave the way to targeting the undruggable proteome
Unfortunately, 85% of the human proteome is considered undruggable because the protein structure is unknown, disordered, or lacking well-defined binding pockets. Many research groups have focused on developing RNA degraders to shift away from this inherent problem with the current protein-centric paradigm of medicinal chemistry. The most clinically successful approach to RNA degradation is with antisense oligonucleotides (ASOs). ASOs are small single-stranded DNA sequences that hybridize to a target mRNA via Watson-Crick-Franklin base pairing and induce the mRNA’s hydrolysis by the ubiquitous enzyme RNAseH. These therapeutics target a disease-associated protein at the transcript level rather than the protein level, enabling degradation of protein targets for which no small molecule inhibitor exists.
A major challenge of all therapeutics, however, including RNA degraders is selectively delivering and activating in target cells while leaving healthy cells alone. These challenges are particularly difficult for ASO development. Indeed, less than 1% of ASOs reach the cytoplasm due to their large molecular weights and the charged nature of the backbone. Moreover, ASOs can hybridize to partially complementary mRNAs causing off-target issues. The Salaita lab has been at the forefront of tackling the delivery, selectivity, and toxicity issues of ASOs by creating cutting-edge drug delivery vehicles, investigating methods to enhance sequence specificity using multivalency, and creating microRNA-responsive ASOs.
Densely functionalized nanodiscoidal nucleic acids potently degrade target mRNA
Delivery of nucleic acids can be hindered by nuclease susceptibility, endosome trapping, and clearance. Multiple nanotechnology scaffolds have offered promising solutions, and among these, lipid-based systems are advantageous because of their high biocompatibility and low toxicity. However, many lipid nanoparticle systems still have issues regarding stability, rapid clearance, and cargo leakage. We have designed lipid nanodisc vehicles that incorporate a modified Apo-A1 mimetic peptide to enable unprecedented DNA loading onto the disc scaffold, which we call nanodiscoidal nucleic acids (NNAs). We demonstrated that these NNAs functionalized with an ASO against HIF1-alpha potently reduce HIF1-alpha mRNA transcript levels compared to ASOs without a vehicle and demonstrated in 3D tumor spheroid models that these NNAs can penetrate into the necrotic core, a substantial challenge of conventional therapeutics. Thus these NNAs potentially offer an improved platform for ASO therapeutics. (ACS Chem. Biol. 2023)
pH-responsive nanoparticles as Trojan Horses to improve ASO drug release into the cytoplasm
One of the biggest challenges facing nucleic acid therapeutics is their inability to escape the endosome, the stomach of the cell. Indeed, 99% of DNA nanotherapeutics are estimated to be destroyed in the endosome before they can engage their target in the cytoplasm. To overcome this limitation, we have designed DNA Endosomal Escape Vehicle Response (DELVR). This new strategy enables DNA nanotherapeutics to escape the endosome by responding to the endosome's acidic environment using pH-responsive i-motif DNA and endosomal escape peptides. We have demonstrated that DELVR substantially increases drug potency compared to traditional nanotherapeutic vehicles and that DELVR mediates this increased potency via increased endosomal escape. Thus, the DELVR concept could offer a solution to the ongoing issue of poor cytosolic delivery of ASOs in viv. (ACS Nano 2024)
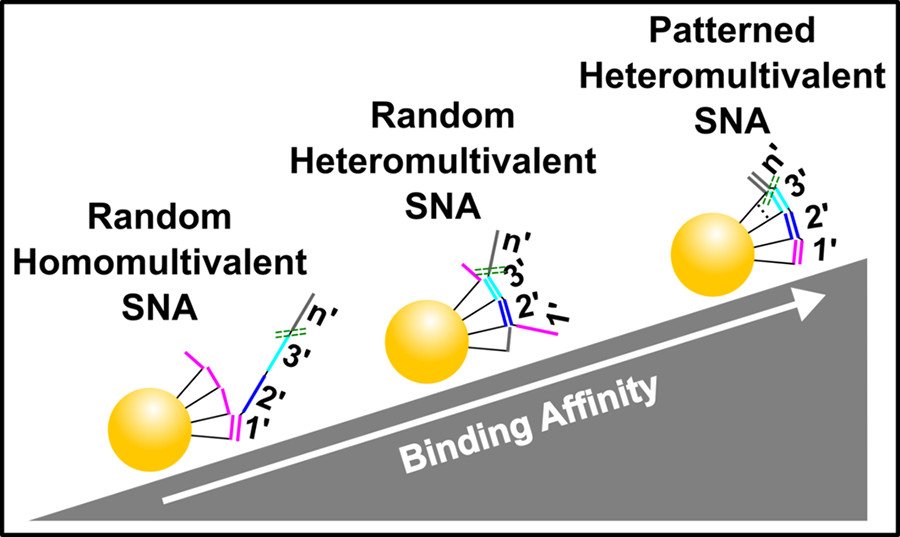
Heteromultivalency leads to enhanced target sequence binding avidity
Improving the affinity of nucleic acids to their complements is an important goal in antisense therapy and diagnostics. One potential approach to achieving this goal is to use multivalent binding, which often boosts the affinity between ligands and receptors, as exemplified by virus–cell binding. Inspired by nature, we have developed spatially patterned heteromultivalent DNA-nanoparticle conjugates and discovered that spatial patterning oligonucleotides on the surface of the nanoparticle drastically boosts target sequence binding avidity and affinity. These insights into DNA hybridization could lead to improvements in hybridization-based disease diagnostic probes and DNA-based therapeutics such as ASOs. (ACS Nano 2020)
Heteromultivalency leads to drastic improvements in binding specificity
Selectively binding to disease-associated single nucleotide polymorphisms (SNPs) is extremely challenging and detecting these mutations is necessary to prescribe effective cancer therapies, perform genetic analyses, and distinguish similar viral strains. Traditionally, SNP sensing uses short oligonucleotide probes that differentially bind the SNP and wild-type targets. However, DNA hybridization-based techniques require precise tuning of the probe’s binding affinity to manage the inherent trade-off between specificity and sensitivity. We have developed heteromultivalent nanoparticles that show extremely high specificity for SNPs and we demonstrated between heterozygous cis and trans mutations and between different strains of the SARS-CoV-2 virus. Our findings indicate that heteromultivalent hybridization offers substantial improvements over conventional monovalent hybridization-based methods. These insights into nucleic acid binding could lead to breakthroughs in SNP treatment and point-of-care diagnostics. (Nature Chem. 2023)
Conditional ASOs that respond to microRNA signals can differentiates between cell types and disease states
MicroRNA (miRNA) are regulatory RNAs that modulate gene expression and are implicated in a variety of cancers and other disease states. Indeed, some miRNAs are exclusively expressed in select tissues or are highly overexpressed (> 10 fold) in diseased tissue compared to healthy tissue. To leverage this tissue and disease-state specificity of miRNA, we have developed conditional ASOs, which can selectively activate in the presence of a trigger miRNA. These conditional ASOs use toehold-mediated strand displacement (TMSD) to peel off the ASO from the locking strand, freeing it up to hybridize to its mRNA target and thus induce its degradation. These results have broad implications in the field of precision medicine and the ASO field at large and could pave the way for next-generation therapeutics with improved specificity and limited systemic toxicity. (ACS Chem. Biol. 2021)
Future directions
Despite our over-decade-long contribution to the field of smart therapeutics, there are still many questions that need to be answered in order to fully harness the power of RNA degraders such as ASOs. For instance, is there a way to create biocompatible vehicles that deliver ASOs and other biologics to organs outside the liver, kidney, and spleen? Is the DELVR platform compatible with lipid nanoparticles and will this change in vehicle cause a change in ASO release? Are there better ways of functionalizing lipid nanoparticles for increased drug loading capacity? What is the fate and uptake mechanism of DNA-functionalized lipid nanodiscs? Would a lipid-based DELVR be well-tolerated in vivo? Would multivalency increase ASO specificity? Can TMSD be used in other ways to improve ASO specificity and potency? These questions and many others are in desperate need of answers, as they could hold the key to a brighter future for those suffering from diseases worldwide.